The Fast Track to Certified Digital Health Products
Embed our MDR IIa medical diagnosis API for a wide range of applications.
Save 1.5-2 years of certification with our ready to use analysis engine.
Reduce the significant costs of certification.
Use your system for medical use cases today.
Made for Digital Health Pioneers
For the MDR IIa-compliant digitalisation of iOT devices, market launch of digital health solutions and CE expansion of lifestyle apps.
For Device Manufacturers
Medical diagnosis of device data.
For Startups
Fast track the certification as a medical product.
For Lifestyle & Wellness Products
Take the next step and offer your solution for medical purposes.

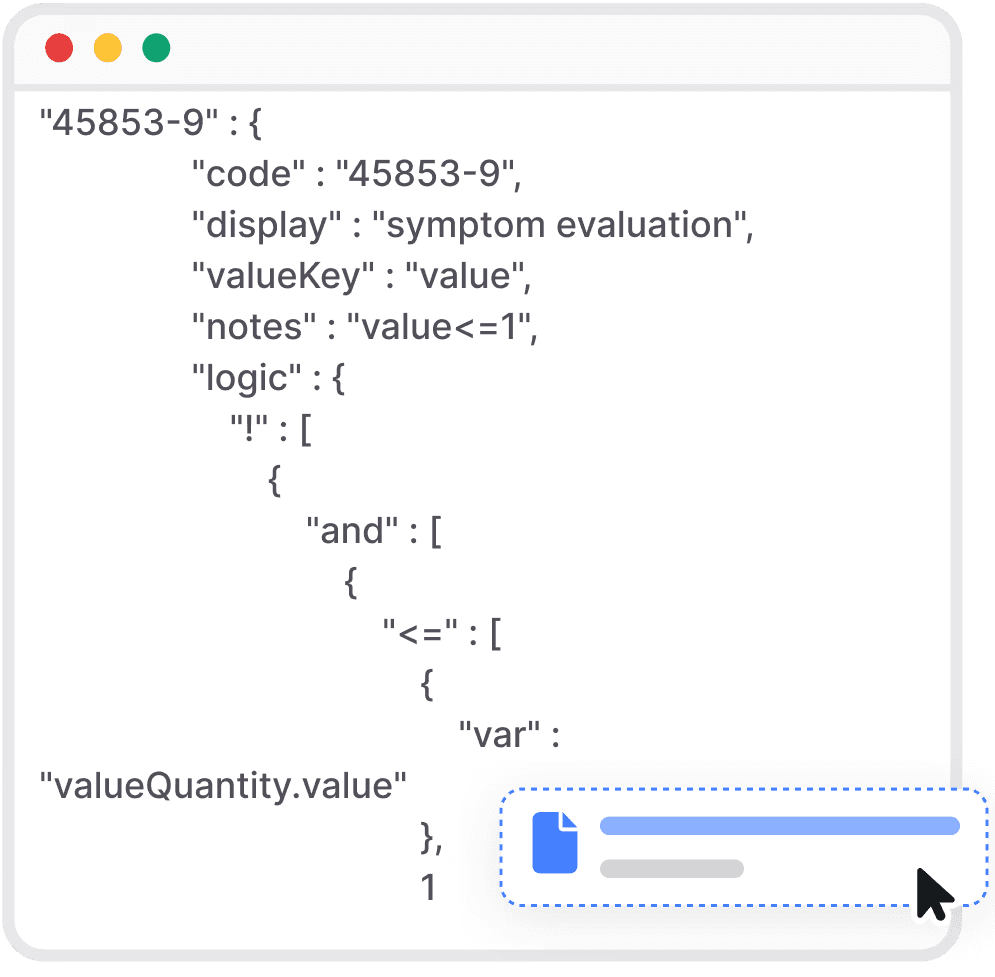
Easy seamless integration of our documented API.
Integrate our API
Check our well documented “API Documentation” and integrate in hours.
Use Your Frontend
To show alerts, patient prioritization and vital parameter values. Or use our Mobile SDK instead.
Provide Your Solution in Medical Environments
Your solution is powered by a clinical, certified engine and ready for doctors and clinics.
What is MDR certification?
Where do I need MDR certification?
Do I even need MDR certification?
What if my product is already MDR-certified, but not for vital signs analysis?
Do I have to become a ‘legal manufacturer’?
Does ACTIMI Signals have a user interface?
How long does it take to integrate ACTIMI Signals?
Are there any restrictions on the use of ACTIMI signals?
What happens if I do not comply with the MDR guidelines?

Julian Charisius
VP Sales
ACTIMI GmbH
Albert-Schäffle St 119
70186 Stuttgart